General vision
To provide methods and tools for mechanistic understanding of the mechanical behavior of biological tissues, to apply this knowledge for uncovering changes due to ageing and disease, and, to exploit this knowledge for diagnostic and therapeutic use for patients at need.
Laboratory Facilities
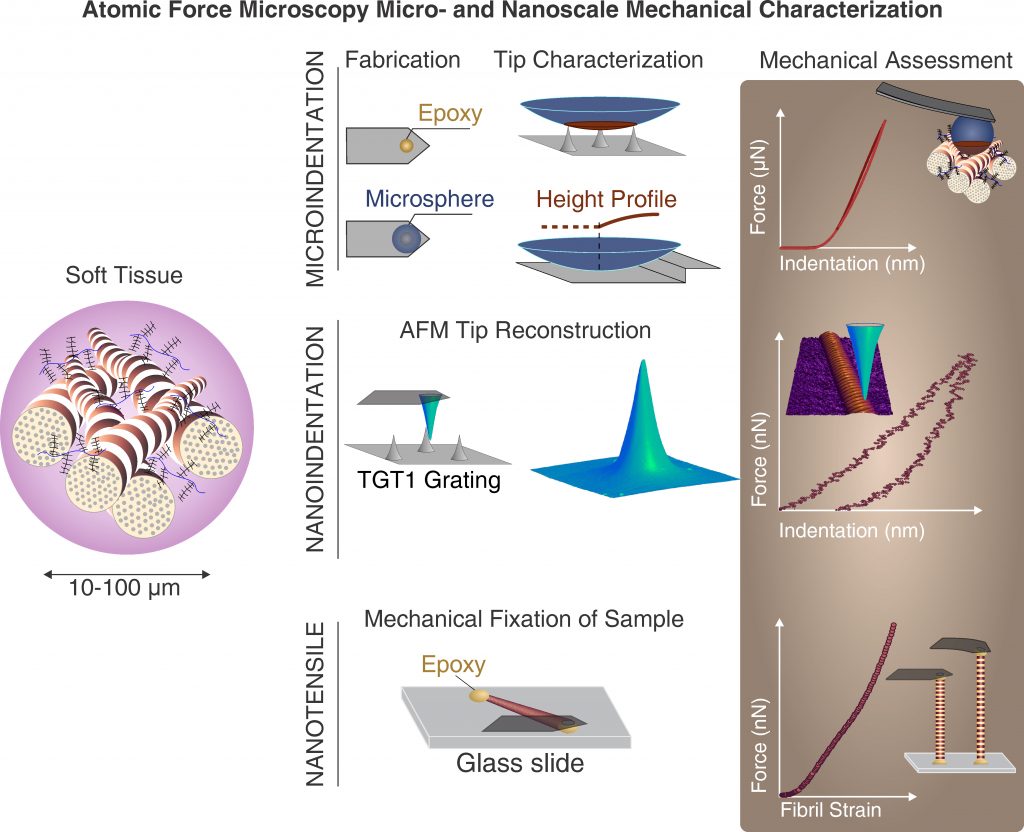
We conduct our experimental studies in the Interfacultary Laboratory for Micro- and Nanomechanics of Biological and Biomimetical Materials (MMLab). We manage the MMLab and are a major stakeholder in it. Our laboratory facilities, are accessible via collaboration arrangements and available methods and expertise include: atomic force microscopy, nanoindentation, micro-computed tomography, light microscopy, micromechanical testing, instrumentation, tissue sample preparation and more.
Multi-scale tissue biomechanics
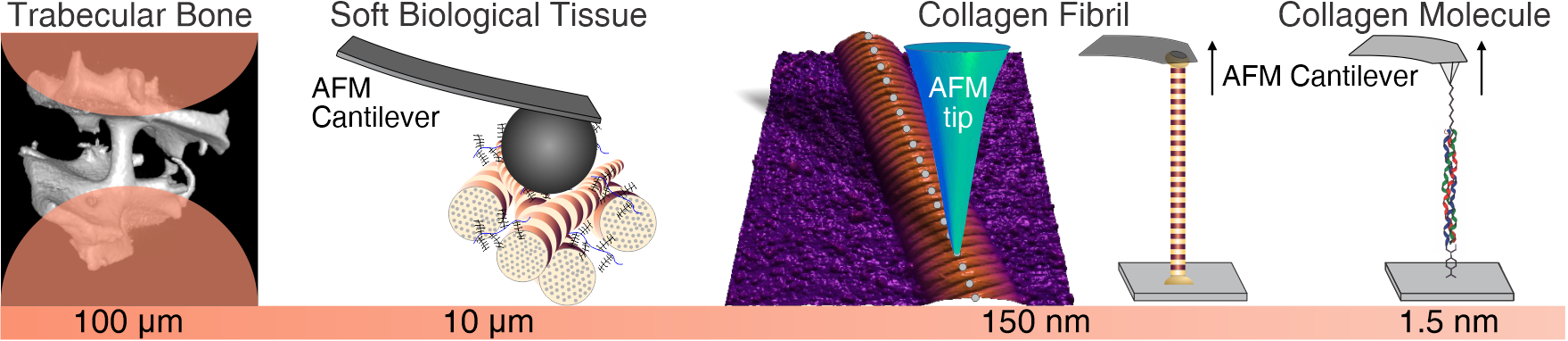
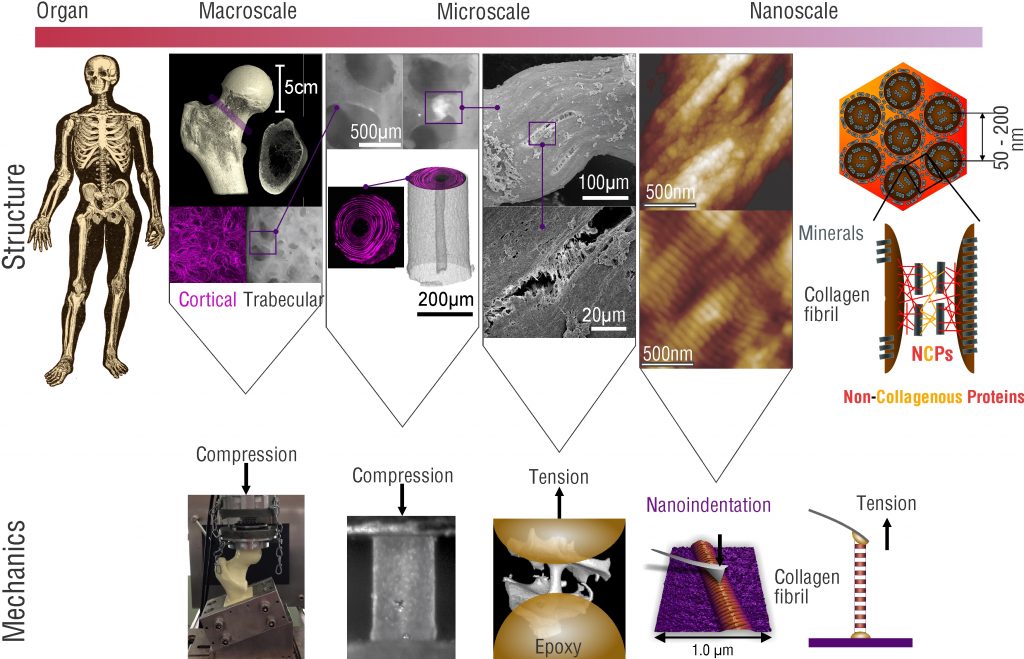
Tissues are by nature multi-scale hierarchical composite materials made from nanoscale building blocks. The underlying reason for the size of these fundamental building blocks arises from the limited ability of biological cells to assemble large monolithic structures. Therefore, the strategy of living systems for provision of macroscale systems such as the human body is to use hierarchical structures. Within these structures the smallest, nanoscale building blocks are composed to form increasingly larger structural elements that finally lead to whole organs and in combination to a complete living system. This highly smart manner of creating giants from dwarfs has, however, intricate consequences for the biomechanics of living systems and the tissues they contain.
Firstly, the definition of material properties, commonly used in material science to define mechanical properties at the material level in a continuum sense becomes a complicated task. Tissues per se behave according to the structures they contain, and, in many cases, it is impossible to define a representative volume element (RVE), As an RVE needs to be much smaller than the structure of interest and much larger than the next smaller structural element.
Secondly, any alteration of mechanical behavior at the macroscale, due to age or disease could principally manifest itself at any scale within the hierarchical structure of the tissue. Therefore, the analysis of what has changed becomes a true challenge, and warrants a multiscale mechanical assessment. As this is generally impossible, the remaining feasible approach is to take an educated guess as to the level of hierarchy, where the changes are expected to be occurring, and to perform experiments at this level.
Within the biomechanics group we follow this approach to investigate the mechanisms by which the mechanical behavior of healthy biological tissues is specified, and, by which changes may occur due to aging and disease. Due to the fact that cells can only “build” rather small objects, this approach also necessitates the abilities to prepare and characterize small samples, that is on the micro- and nanoscale. For such purposes, miniature testing systems and custom approaches have been and are constantly developed to provide mechanistic understanding of tissue biomechanics. Research in this area is focused on changes in the mechanical properties of hard- (bone) and soft tissues as a function of age and pathology.